-013 1x 8 4pts For the reaction between magnesium and oxygen describe the reactant appearance and identify the. The unbalanced reaction between magnesium and oxygen is.
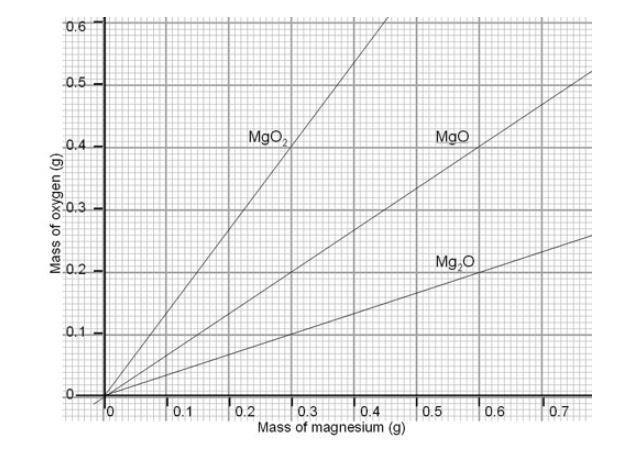
The Change In Mass When Magnesium Burns Experiment Rsc Education
Its endothermic with the reactantsThe total first and second ionization enthalpies for Magnesiums 3s 2 electrons are H.

. The combination reaction between magnesium and oxygen with word and balanced symbol equationsThis video would be useful for those studying the NCEA Chemistr. An instance of a chemical reaction is the burn of methane in the visibility of molecular oxygen O2 to kind carbon dioxide CO2 and water. Oxygen is in group 6 of the periodic table.
Actually the oxidationcombustion reaction for MgO is an endoexo thermic reaction. REPORT SUMMARY Reaction of Magnesium and Oxygen The video below shows the reaction of magnesium and oxygen the black solid that remains after the reaction falls away. B They form an ionic bond by exchanging three electrons.
The reactions with oxygen. Atomic number of magnesium is 12 and its electronic configuration is 2 8 2. D They form a covalent bond by sharing four electrons.
You will often see electrons drawn like this in books. The chemical reaction beyween magnesium and oxygen is given below. 742 and 1450 kilo Joules mole.
However unlike magnesium sodium reacts immediately when it is exposed to oxygen and produces very little heat and no visible light. The students observe bubbles forming on the surface of the metal and the solution turns white. Δ is heat taken by Mg to react with O 2.
An oxygen atom will gain 2 electrons to form a stable 2-ion. Chemistry questions and answers. Attachment Reactant Reactant appearance Evidence of chemical reaction Mg s Balance.
In Lesson 20 a magnesium strip was used to ignite the thermite reaction. Atomic number of oxygen is 8 and its electronic configuration is 2 6. When magnesium is placed in a flame from a small blow torch it burns quite brightly forming magnesium oxide a salt that contains one magnesium for every oxygen.
Then balance the chemical equation and identify the type of reaction. We can go further and translate the picture equation for the reaction between magnesium and oxygen to a chemical equation. Use the video to answer the following questions.
In this example the electrons are shown as dots and crosses. For the reaction between magnesium and oxygen describe the reactant appearance and identify the evidence of chemical reaction. M gO2 M gO M g O 2 M g O.
Write a balanced chemical equation to represent this reaction. Magnesium reacts with oxygen to make a compound called magnesium oxide. Sodium will also react with oxygen.
C They form a covalent bond by sharing one electron. In the following questions you will be asked to provide some details of the chemical processes involved in a reaction between magnesium. The reaction of magnesium and oxygen can be said as burning the magnesium.
2 Mg O2 2 MgO. The following questions ask you to provide some details of the chemical processes involved in a reaction between magnesium and oxygen. A They form an ionic bond by exchanging two electrons.
A group of students wants to observe the reaction between magnesium metal and hydrochloric acid. Fire is what we call the heat and light produced when things burn. Hence an ionic bond will be formed.
Part A of Redox Reactions Lab. Magnesium gives up two electrons to oxygen atoms to form this powdery product. 2MgO 2 Δ ---------- 2MgO.
They place a 12-g strip of the metal into a test tube containing 47 g of aqueous hydrochloric acid. A magnesium atom will lose 2 electrons to form a stable 2 ion. 2 question The reaction between magnesium Mg and oxygen O2 forms magnesium oxide MgO.
Which best describes how magnesium Mg and oxygen O bond. Thus we can conclude that the statement they form an ionic bond by exchanging. CH4 2O2 CO2 2H2O In this reaction which is an instance of a burning reaction changes occur in the means that the carbon hydrogen and oxygen atoms room bound together in the compounds.
2 Mg O 2 2 MgO Since the chemical equation consists of symbols we can think of this as a symbolic representation. An energy diagram for the magnesium-oxygen reaction is shown to the right. The ionic bond between magnesium and oxygen is stronger than the ionic bond between.
When magnesium burns it is actually reacting with oxygen in the air and not with fire. So in order to attain stability magnesium will lose two electrons and oxygen will gain two electrons.

Which Best Describes This Reaction Between Magnesium And Oxygen Decomposition And Redox Reactions Brainly Com
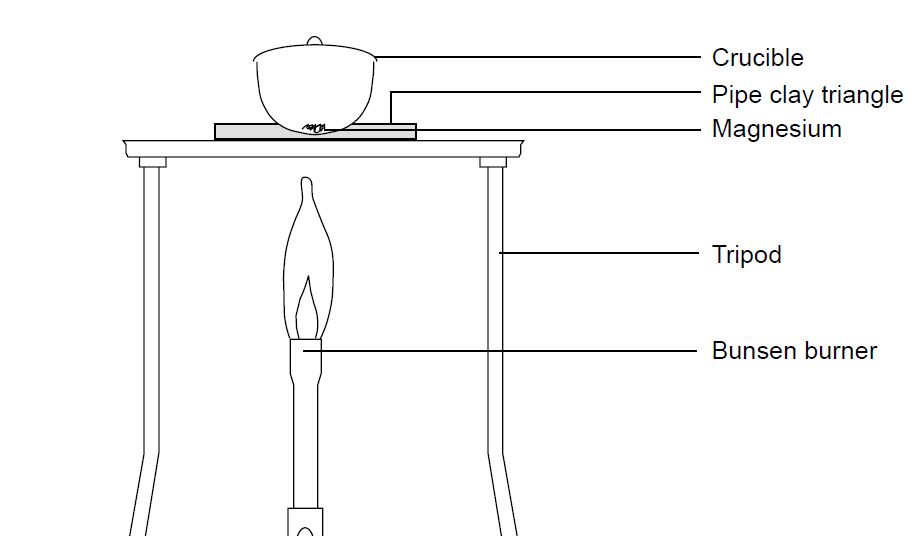
The Change In Mass When Magnesium Burns Experiment Rsc Education

The Law Of Conservation Of Mass Can Be Demonstrated By A Chemical Reaction Which Of The Following Brainly Com
0 Comments